Crude oil to base stocks to base oils to lubricating oils
The refining process has improved throughout the history of crude oil use, with base stock quality continuing to benefit from new technology.
Lubrication insights
- Refining separates the crude oil into useful products by distillation and the crude is pumped into the bottom of a distillation tower and heated to volatize the lighter, or smaller molecules.
- Base stock quality continues to benefit from new technology and the vacuum distillation process helped convert a byproduct into a useful base stock for lubricants.
Crude oil was produced from decayed organic matter over millions of years under pressure and heat. Common thought is that the organic matter decomposed into crude oil was dinosaurs. Although dinosaurs may have contributed, the major source was bacteria from decaying dead vegetation along with various animals, marine life, and lizards (dinosaurs) primarily at the bottoms of oceans and inland waterways. Sediment, dirt, and rock deposits buried the organic matter and created the pressure to compress the decayed matter into liquid mixtures. Due to this environment, the crude oil is not pure hydrogen and carbon from the carbon-based life forms but contains impurities such as water, sulfur, oxygen, and nitrogen, to name a few.
The crude oil was thus captured in porous rock formations from which it can be extracted. The term “petroleum” is derived from Medieval Latin “petra” meaning rock and “oleum” meaning oil. Typically, crude oil and natural gas are found together in these rock formations, although either may be found separate from the other. The natural gas, separated from the crude oil, is piped from the well site and sold as natural gas after some additional clarification of the various components, such as methane (primary component of natural gas), ethane, propane, and butane. The liquid crude oil from the oil well is shipped to refineries for processing.
Refining separates the crude oil into useful products by distillation. The crude oil is pumped into the bottom of a distillation tower and heated to volatize the lighter, or smaller molecules. The lighter gases rise up the distillation tower, cooling as they rise. As they cool, they become liquid again and are removed from the distillation tower. Roughly speaking, the first familiar liquid removed might be heavy fuel oil, typically used in ocean-going ships and power stations. The next liquid could be lubricant base stock, then diesel, followed by kerosene and aircraft fuel. Naphtha used for making chemicals and then gasoline for your cars would commonly come out next. Finally, “bottle gas” comes off the top of the distillation tower.
The ambiguity previously mentioned on the products coming from the distillation tower was necessary because the composition of the crude oil dictates the refining process and to a great extent what products are to be made from the crude oil. The composition of the crude oil is due to the type of organic matter and the historic geology during formation. Crude oils formed from non-marine sources, such as in the Far East and Central Africa, are similar to each other but different than oils from Nigeria and Louisiana that were formed from similar marine life.
The primary product of the first oil wells and distilling was kerosine for illumination before the invention of the light bulb. The heavy dark liquid from the well was a byproduct of little value. The rapidly expanded use of the electric light bulb nearly killed the budding petroleum industry. Fortunately, the advent of the automobile saved the industry with its thirst for gasoline and made the byproduct valuable as motor oil.
In the U.S., and maybe beyond its shores, Pennsylvania Grade Crude Oil is a marketing term still occasionally used today. It is derived from the actual Pennsylvania Crude Oil that was discovered in the mid-19th Century. The crude oil was high in alkanes (straight saturated hydrocarbon chain molecules that we’ll discuss later) that resist oxidation and has a relatively high viscosity index, making it good for lubrication with minimal distillation. Other oil fields around the world have been discovered with similar properties, with some labeled “sweeter” than others. Pennsylvania Crude Oils with 19th Century distillation capabilities were regarded as high-quality motor oils then but could not meet the demands of today’s motor oils.
Oil fields with more complex components that were neither as stable nor made as good of a lubricant required more sophisticated refining methods to produce the alkanes in the right range for lubricating oils. Early distillation towers operated at atmospheric pressure and had a practical limit of approximately 650 °F. At higher temperatures, crude oil thermally decomposes. To further process or segregate the various components of the crude oil into useful liquids, the distillation tower is operated under a vacuum. The vacuum effectively lowers the vaporization temperature of the crude oil so the different size crude oil molecules, particularly the size molecules used for lubricants, and larger, can be separated in the distillation process without breaking down the compounds.
Although the makeup of the product slate is partially dictated by the type of crude oil, the market demand also drives invention. Lubricants might be more easily made from sweet crudes, but with today’s refining technologies, gasoline and lubricants might even be made with the crude oil from the likes of the “tar pits” of Southern California. That may not be economically prudent, though.
The importance of base stocks
Base stock quality continues to benefit from new technology. The vacuum distillation process helped convert a byproduct into a useful base stock for lubricants. Since then, solvent dewaxing helped lower the pour points of lubricants, which was upstaged by catalytic dewaxing. Hydrogen processing helped improve oxidation resistance and high pressure hydrotreating further reduced oxidation tendencies. Hydrocracking (isomerization) enabled the refiners to convert certain crude oil molecules into desired lubricant or fuel molecules.
The American Petroleum Institute (API) recognized base stocks on the market could vary greatly in chemical and physical properties that provided inconsistent engine oil performance. The lubricant market had become a world market where certain performance characteristics needed to be consistent wherever the lubricant was manufactured. Engine oil manufactures had legitimate need for flexibility in base stocks used in lubricants produced in different regions of the world. In 1993, to help with Base Oil Interchangeability, or fungibility, the API introduced five main groups of base stock categories. API further defined a minimum physical and engine testing to determine no adverse effect from substitution of one base stock for another. Before the additional engine testing, the physical properties of the substituted base stock needed to be met by the replacement base stock. The primary base stock properties tested are:
-
Kinematic viscosity at 100 C, 40 C and -40 C
-
Viscosity index (VI) (defines viscosity change with temperature; higher VI means less viscosity change with temperature changes)
-
NOACK volatility
-
Pour point
-
Unsaturates.
Before we go any further, we’ll need to discuss some very limited chemistry. We mentioned the high alkane content of the Pennsylvania Crude Oil. Alkanes are composed of carbon and hydrogen where the number of hydrogen atoms is two more than twice the number of carbon atoms. Methane has one carbon atom and four (2 + 2 x carbon) hydrogen atoms. Propane has three carbon atoms and (2 + 2 x 3 carbon) eight hydrogen atoms. Lubricant molecules typically have 10, 20 and more carbon molecules. When we look at a saturated alkane, it has the same ratio of carbon to hydrogen atoms and is generally a straight paraffinic molecule as shown in Figure 1. If a couple of the hydrogen atoms were missing, or substituted with another atom, it would no longer be saturated and would be more susceptible to oxidation, or molecular breakdown. Ideal lubricants made of saturated hydrocarbons are resistant to oxidation or molecular breakdown, whereas our bodies prefer to consume unsaturated carbs, or fats, because our body can break them down and process them for energy. Saturated hydrocarbons are bad for our bodies, but they are good for lubricants. If any of the hydrogen atoms are replaced with another atom (such as oxygen, nitrogen or sulfur) or another group of atoms not of hydrogen or carbon, the molecule becomes unsaturated and more susceptible to reaction or degradation.

Figure 1. Saturated hydrocarbon molecule, paraffinic.
Solvent dewaxing was part of the refining process that lowered the pour point of the base stock, and yes, paraffin wax comes from petroleum. Straight paraffins, wax, solidifies at relatively mild temperatures and is not ideal for cold climate lubrication, thus the need to remove the wax. Catalytic dewaxing, introduced in the 1990s, converts these straight hydrocarbon molecules to branched hydrocarbons, still saturated and still stable as a lubricant base stock (see Figure 2).

Figure 2. Saturated “normal,” paraffin and isoparaffin. Courtesy: STLE
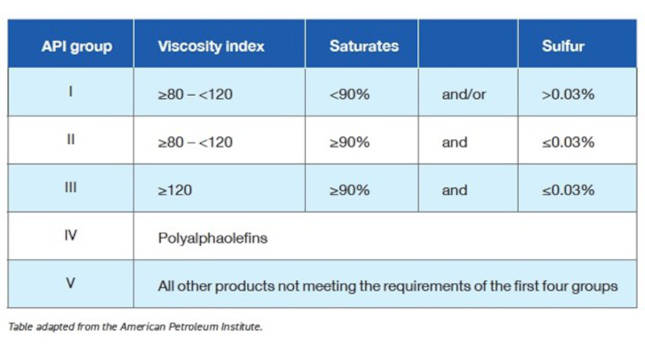
API Group I base stocks have less than 90% saturates, i.e., more than 10% unsaturates. If the base stock contains more than 0.03% sulfur, it would be categorized as a Group I base stock, also. Finally, the base stocks viscosity index (VI) must be greater than or equal to 80 and less than 120. API Group I base stocks are the product of solvent refining introduced in the 1930s.
API Group II base stocks have 90% or more saturates and less than or equal to 0.03% sulfur. The VI must be greater than or equal to 80 and less than 120, just like API Group I. API Group II base stocks may be produced by hydrocracking and catalytic dewaxing to remove the impurities.
API Group III base stocks have 90% or more saturates and less than or equal to 0.03% sulfur, similar to Group II, but has a VI greater than or equal to 120. API Group III base stocks are manufactured utilizing hydrocracking, catalytic dewaxing and hydrotreating to remove impurities and raise the VI.
API Group I and II are considered mineral oils. API Group III may be called synthetic oil by some companies because of the synthetic-like performance. Others call it mineral oil because it is refined from crude oil.
API Group IV base stocks are polyalphaolefin (PAO) synthetic oils with a VI range of 125-200. These base stocks are synthesized versus refined from crude oil.
API Group V base stocks are any other base stock not covered by API Groups I, II, III or IV, such as naphthenic oils, esters, polyglycols, silicones, biolubes and halogenated fluids. If a fluid is a synthetic fluid but is not a PAO, it would be an API Group V base stock (see Table 1).

Table 1. API base oil classifications. Courtesy: STLE
Base oils are the platform on which lubricants are built. A base oil is a blend of base stocks, or occasionally a single base stock, to which additives are added to create a lubricant. It provides the lubricant film and the minimum viscosity. The base oil is the major component of lubricating oils even in engine oils, which have a much higher percentage of additives than gear, hydraulic or turbine oils. Lubricant additives will have to be the subject of another article.
So, in this era dominated by hydrotreated Group II and Group III base stocks, the notion of “Pennsylvania Grade Crude” becomes irrelevant.
Reprinted with permission from the August 2022 issue of TLT, the official monthly magazine of the Society of Tribologists and Lubrication Engineers, an international not-for-profit professional society headquartered in Park Ridge, Ill., www.stle.org. STLE is a CFE Media and Technology content partner.
Original content can be found at STLE.
Do you have experience and expertise with the topics mentioned in this content? You should consider contributing to our CFE Media editorial team and getting the recognition you and your company deserve. Click here to start this process.